Daniel Giannetti
The need for an artificial heart like BiVACOR
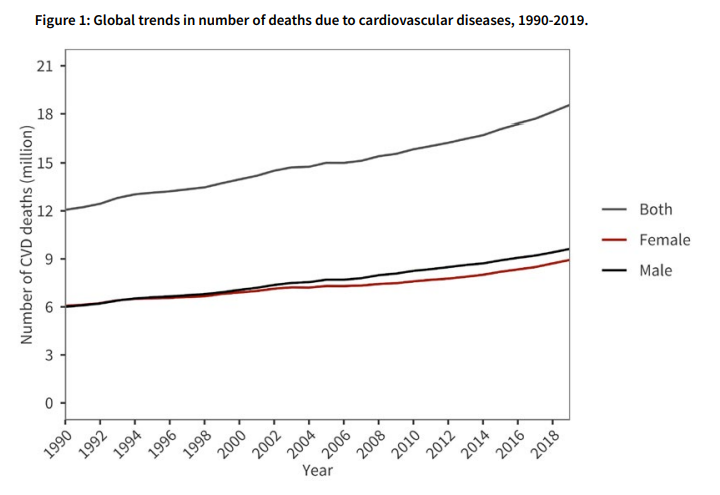
Heart disease remains one of the leading causes of death globally. Heart failure in particular affects at least 26 million people worldwide per year, and heart transplants are limited to those with severe heart failure with millions of others left waiting. It is the goal of many biotechnologists to find a solution to this global challenge, whether that be through regenerative stem cell-based medicine or xenotransplantation – seen recently in transplanted pig hearts. BiVACOR may be the solution to this challenge – a total artificial heart that utilises a single motor with MAGLEV technology to pump blood to the lungs and body.
What is BiVACOR?
At its core, BiVACOR is an artificial heart designed to completely replace a failing human heart. Traditional mechanical hearts have utilised volume displacement pumps (VDPs) with flexible membranes and artificial valves to pump blood. These have suffered in the past from poor long-term durability and have been limited to 2 years of use. Innovative rotary devices have been in development in recent years to overcome these durability issues. Rotary blood pumps generally have less moving parts which means less wear on key components and a longer service life.
BiVACOR is one of these rotary-based total artificial hearts (TAH), which aims to do the heart’s entire job by using a motor and rotating disk suspended between magnetic fields (MAGLEV). This allows BiVACOR to pump blood continuously throughout the body, just like a real heart does, i.e. without the need for flexible membranes or other parts in motion that may wear out over time.
What Are the Challenges?
Though BiVACOR is a promising breakthrough, it’s still experimental and has yet to be used in humans. The road from laboratory development to clinical use for a medtech device is long and requires rigorous testing to ensure a device’s safety and effectiveness. It is very similar to the Drug Development Pipeline, which we have touched on in many previous articles (read here). There are also challenges related to long-term durability and how the human body responds to continuous blood flow, which is different from a typical heart’s pulsed flow. Some studies suggest that continuous flow may be a more efficient way to pump blood around the body and to the lungs, though further investigation will be required to determine the extent of this potential benefit – especially in human trials.
So... How Does It Work?
The BiVACOR artificial heart is manufactured with titanium for all blood and tissue contacting surfaces to ensure that the device is biocompatible. Blood is pumped continuously by a motor and can support flow rates consistent to a normal heart for both rest and moderate exercise (3 to 12L/min). The MAGLEV technology in the rotor eliminates any mechanical contact between the rotor and the casing minimising mechanical wear and blood trauma which has been a persistent problem in past devices. The device adapts to physiological changes to support various patient conditions and activities by adaptively modifying overall flow rate, pulsatility, and balance between left and right output flow to best support a patients day to day activities (similar to a normal heart!).
What's the catch?
This is where the fun dulls slightly. BiVACOR is powered by a driveline which is a percutaneous wire that connects the implanted device to an external control module. The control module is powered by two rechargeable batteries that have roughly a 6-hour run time – YIKES. The control module does have a loud speaker and a display which is essential considering in its absence a low battery powernap may prove fatal! The driveline itself is also a concern as it exits the skin near the abdomen which has the potential to lead to infection. The patient will also have to lug around this control unit with them at all times limiting a patient’s mobility and freedom. These limitations are much less of a burden when compared to previous devices, and can be somewhat overlooked as the unfortunate alternative is to continue to suffer from heart failure until a transplant is available.
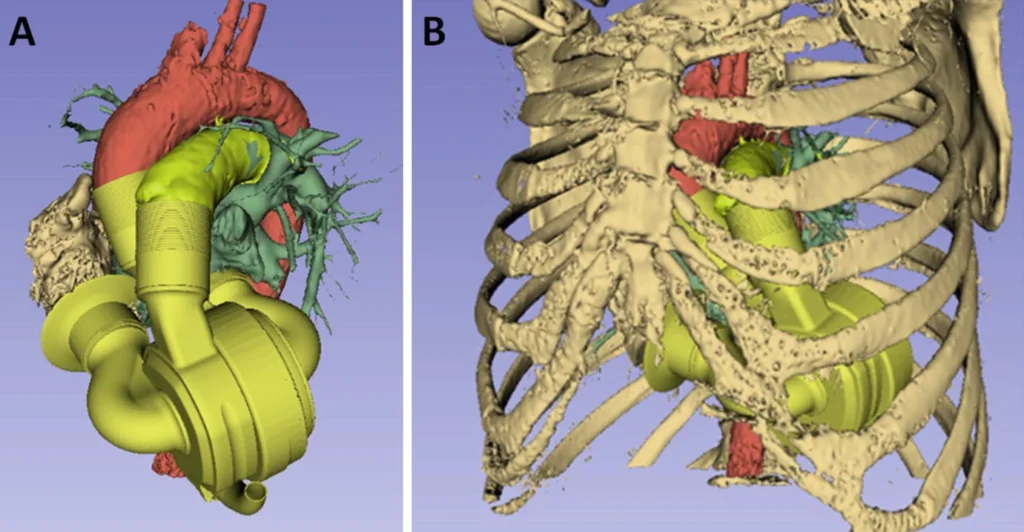
The wait for feasability will not be long
Luckily, BiVACOR have begun human trials already! Following the announcement on their website, the successful first-in-human implantation of the BiVACOR (TAH) has commenced as part of the U.S. Food and Drug Administration (FDA) Early Feasibility Study (EFS) on July 9, 2024. The clinical study aims to evaluate the safety and performance of the BiVACOR device. BiVACOR is being positioned as a short term solution that will act as a quality of life enhancement for a patient suffering from heart failure until they are able to receive transplant. It is safe to say we will be watching this trial very closely!
The Bottom line
BiVACOR offers a remarkable improvement over current implantable heart technologies and is currently trialling the device in humans. Time will tell if this device becomes the industry standard. Regardless, the device is a step in the right direction to ease the burden on the vast number of patients waiting for a heart transplant. It will be interesting to see how the human trials develop and what advancements the company may make in the device itself.