Antibiotics are arguably one of the most important biotechnological inventions of the last century. Access to effective antibiotic and antimicrobial drugs has saved countless lives which has allowed for advancements in healthcare, increasing the success of other medical interventions such as surgery and chemotherapy.
However, resistance to current antibiotic drugs is a serious issue on the verge of becoming a global health catastrophe if novel solutions are not discovered. According to the World Health Organisation (WHO), the most prominent AMR is visualised between pathogenic bacteria and antibiotics.
In our May Newsletter, we discussed the next big biotech breakthroughs, one of them being a novel antibiotic. This article will dive deeper into the need for a novel antibiotic and scientists’ key challenges in creating one.
In This Article..
When we talk about the first antibiotic drug, we are discussing the first intervention used to treat infections at a time when scientists understood that microbes such as bacteria-caused these infections. However, it is important to note that ancient civilisations used plant and mould pastes for their antimicrobial properties before this knowledge.
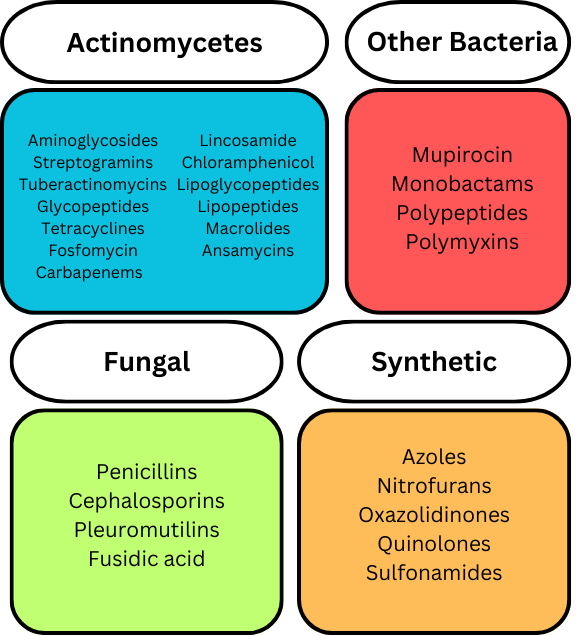
There are multiple classes of antibiotics, and the classification of antibiotics can be based on different aspects. Antibiotics can be classified by how they were created, how they function, where they function, what (microorganisms) they were derived from, or if they are synthetic. A basic summary of some different antibiotic drugs, classified by their origin, is shown below.
A Brief History of Antibiotics
1900 – 1940: Antibiotics for Clinical Use
A significant moment in the development of antibiotics was in 1910 when Salvarsan was released. Salvarsan was the first synthetic antibiotic drug and was derived from dyes. However, anti-microbial resistance to salvarsan was prevalent by 1930 resulting in a phasing out of the clinical use. A similar story was seen with the introduction of sulfonamides, another group of synthetic antibiotics released in the 1930s. It only took a decade for resistance to occur.
1940s – 1960s: The Golden Era of Antibiotics
The discovery of penicillin in 1928 by Alexander Fleming was a significant turning point in the success of antibiotics. Penicillin was hailed a ‘wonder drug’ when it was finally brought to the market by Howard Florey and Ernst Chain in 1941. At a time when infections such as pneumonia and rheumatic fever were incurable, and in the later stages of WWII when people were still dying of blood poisoning from cuts and septicaemia, penicillin was a life-saving drug. Additionally, penicillin was a more effective antimicrobial drug and caused fewer side-effects than earlier antimicrobials such as salvarsan and sulfonamides.
1960s – 2010: Antibiotic Resistance Continues to Rise
Following the Golden Era of Antibiotics, a multitude of other prominent antibiotic drugs entered the market, including over 20 new classes of antibiotics. Unfortunately, antibiotic resistance also increased, with scientists discovering Methicillin-Resistant S. aureus (MRSA) in 1961.
Although some existing antibiotics were ‘redeveloped’ and new antibiotics were discovered, AMR continued to progress and by 2010 vancomycin-resistant enterococci (VRE), vancomycin-resistant S. aureus (VRSA) and plasmid-borne colistin resistance in Enterobacteriaceae was uncovered and identified as critical threats.
2010: AMR Declared A Fundamental Threat
The World Health Organisation Declared Antimicrobial Resistances a ‘Fundamental Threat’. This led to the WHO formulating its Bacterial Priority Pathogen List to highlight crucial areas in which scientists could generate novel antibiotic drugs.
How Does Antimicrobial Resistance Occur?
Antimicrobial resistance can occur due to several factors. Often, people assume the obvious cause is when microbes such as bacteria, viruses and fungi evolve to become resistant or immune to antimicrobial therapies, due to mutations or immune response development.
However, taking a step back, the most prominent cause of antimicrobial resistance is the misuse or overuse of antimicrobial drugs. This misuse of drugs such as antibiotics allows these microbes to adapt and establish resistance and reproduce these resistance strains of microbes. Misuse refers to overconsumption or overprescribing antibiotics to treat infections that may be treated using alternative interventions. Additionally, the overuse of single antibiotic classes in single patients instead of alternative antibiotics, which may target different aspects of the disease, can lead to increased antimicrobial resistance.
What Are The Consequences?
Public Health Threat: Resistance to antibiotics poses a significant global health threat. According to the World Health Organisation (WHO), bacterial AMR is one of the top public health and development threats responsible for over 1.27 million global deaths in 2019. Additionally, AMR can cause issues in other sectors of healthcare, most prominently with surgical procedures.
Economic Burden: In terms of the economic burden, The World Bank estimates that AMR could cost the healthcare system an additional US$1 trillion by 2050. This cost may seem significant for countries with sophisticated healthcare systems, however, the true burden of this would be felt by people from lower socioeconomic backgrounds and developing countries.
What Now?
The World Health Organisation recently released an update on the AMR crisis and current therapies in development to combat the WHO Bacterial Priority Pathogen List.
- Preclinical: 244 products are currently in preclinical development targeting WHO 2023 Bacterial Pathogenic Priority and C. difficile. These are being developed by 141 individual developers/groups.
- Clinical: 97 antibacterial agents and/or combinations (containing at least one novel therapeutic agent) are currently in the clinical trial antibacterial pipeline. From these, 57 are traditional antibacterial therapies and 40 are novel (non-traditional) agents.
- Approved: 16 new antibacterial therapies have been approved by the FDA since July 2017. A very low number of novel therapies to treat this crisis.
Overall, the collaboration between the scientific community and the World Health Organisation is working to combat the AMR crisis. Without the active monitoring and management by the WHO, and the scientific community’s recognition of their recommendations, AMR has the potential to have catastrophic consequences. However, as you hopefully have learnt by now, the drug development pipeline is a long, treacherous, and expensive journey, and the low success rates are to be expected.
An excerpt from the WHO Fact Sheet on Antimicrobial Resistance highlights the urgency of this situation.
“The world faces an antibiotics pipeline and access crisis. There is an inadequate research and development pipeline in the face of rising levels of resistance, and urgent need for additional measures to ensure equitable access to new and existing vaccines, diagnostics and medicines.”
World Health Organisation, 2024
Extra Reading
For those interested in this topic, I’ve included some links for further reading.
Antibiotics: past, present and future
WHO Antimicrobial Resistance Fact Sheet
2023 Antibacterial agents in clinical and preclinical development: an overview and analysis
Find out more about MRSA and VRSA, in our August Newsletter and Podcast
